|
Up to 9 missed cleavage sites can be allowed, specified by
the Missed Cleavages parameter.
"None" means that Mascot will search each protein sequence
for every sub-sequence which meets the other search criteria.
This usually means testing orders of magnitude more peptides than if trypsin cleavage
had been specified.
If the experimental data are from peptides that do not originate from an enzyme
digest, such as MHC peptides, then "None" is the correct choice.
Otherwise, ignoring the enzyme specificity greatly increases both the search time and,
more importantly, the identity threshold,
which will usually result in fewer significant matches in total. An
error tolerant
search is a much better way to identify the occasional non-specific cleavage product.
"None" is never allowed
for a Peptide Mass Fingerprint, where
the specificity of an enzyme is essential.
"semiTrypsin" means that Mascot will search for peptides that
show tryptic specificity (KR not P) at one terminus, but where the other terminus may
be a non-tryptic cleavage. This is a half-way house between choosing
"Trypsin" and "None". It will only fail to find peptides
that are non-specific at both ends.
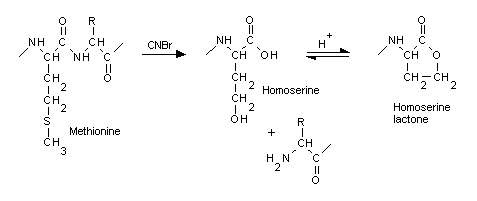
When a protein is cleaved by the commonly used enzymes, the
new C terminus gains a hydroxyl group, while the new N terminus
gains a hydrogen. The activity of cyanogen bromide (CNBr) is unusual
in that cleaves on the C-terminal side of methionine, converting
it to a homoserine. Under acidic conditions, homoserine generally
cyclises to the lactone form, which does not require any terminating
group. In Mascot, when CNBr is chosen as the cleavage agent, you also need to specify
homoserine (Met->Hse) or homoserine lactone (Met->Hsl) as variable modifications.
Peptide Size Distribution
If we make the (fairly accurate) assumption that the proteins
in a database can be treated as composed of truly random sequences
of the 20 standard amino acids, then it is straightforward to
calculate the peptide size distribution to be expected from any
given enzyme.
In the following graph, peptide size distributions are plotted
for limit digests of chymotrypsin (assumed to cleave at FWYLMH),
trypsin, and CNBr:
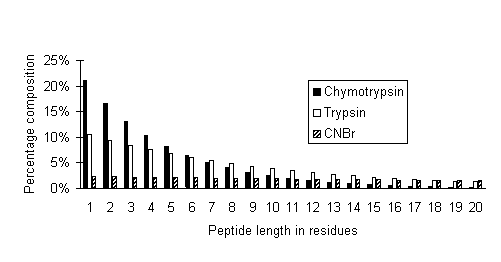
It can be seen that the high specificity cleavage reagent,
CNBr, produces a relatively flat distribution of peptide sizes,
ideal for fingerprinting by MALDI. In the case of the low specificity
enzyme, chymotrypsin, less than 1% of all peptides are longer
than 20 residues and 70% are between 1 and 5 residues. In a mass
spectrum, peaks for these peptides would be crowded together into
the low mass region, resulting in extensive overlapping.
Trypsin is somewhere in between, with 10% of peptides longer
than 20 residues. Because of the relative scarcity of these larger
peptides, it is the higher experimental mass values which provide
greatest discrimination in a peptide mass fingerprint.
|